Dt&CRO
Research Centers
Our creativity and passion drives our dream of becoming the future of CRO.
IT Services

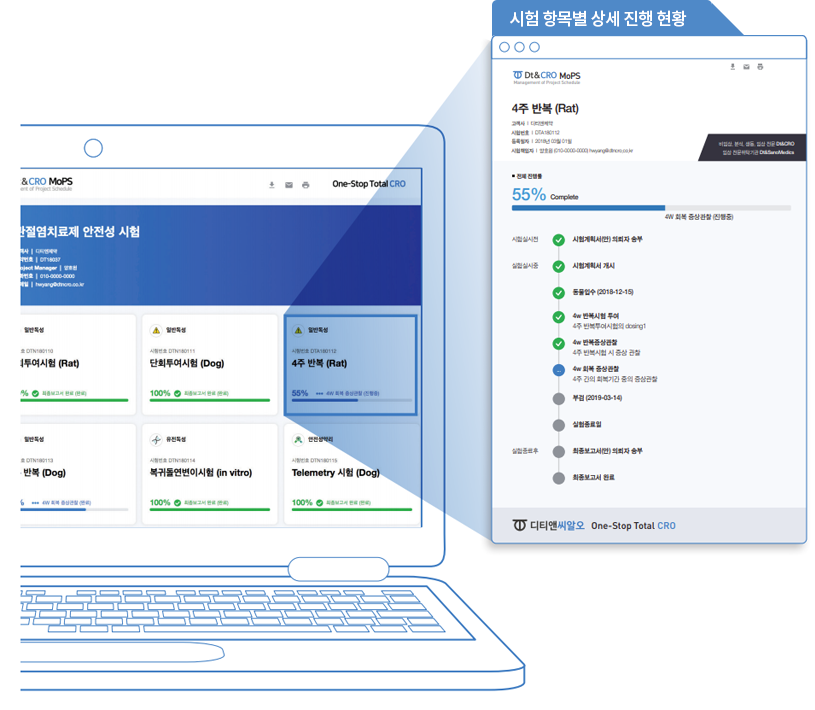
MoPS
Management of Project Schedule
Our real-time project schedule managing program MoPS enables seamless communication with sponsors,
allowing them to track phased progress in real-time via web email.
Collaborate efficiently and share progress internally with ease.

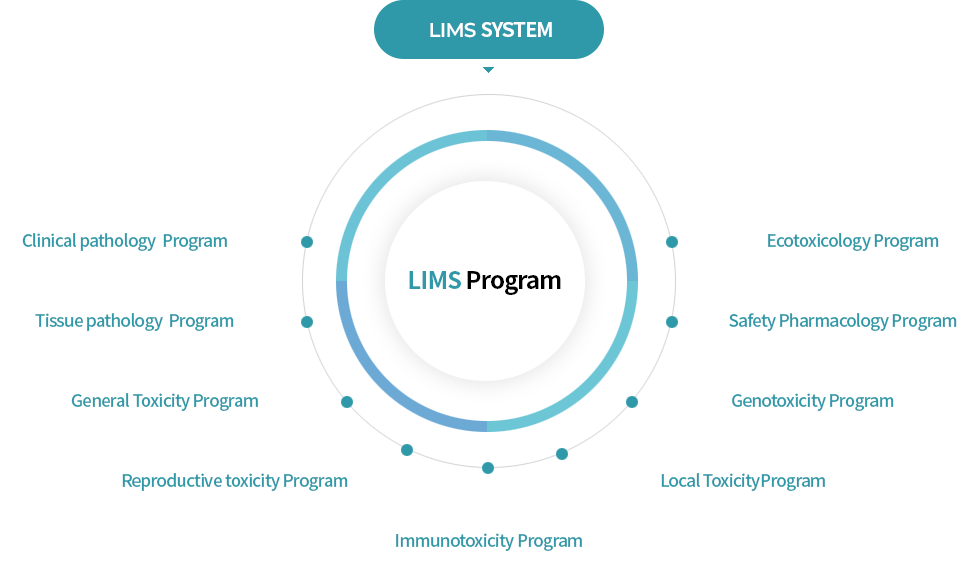
LIMS
Laboratory Information Management System
Enhance study reliability with our Laboratory Information Management System (LIMS).
LIMS automatically connects to SEND (Standard for Exchange of Nonclinical Data) for U.S. FDA IND or NDA approval, ensuring accurate data exchange for regulatory compliance.

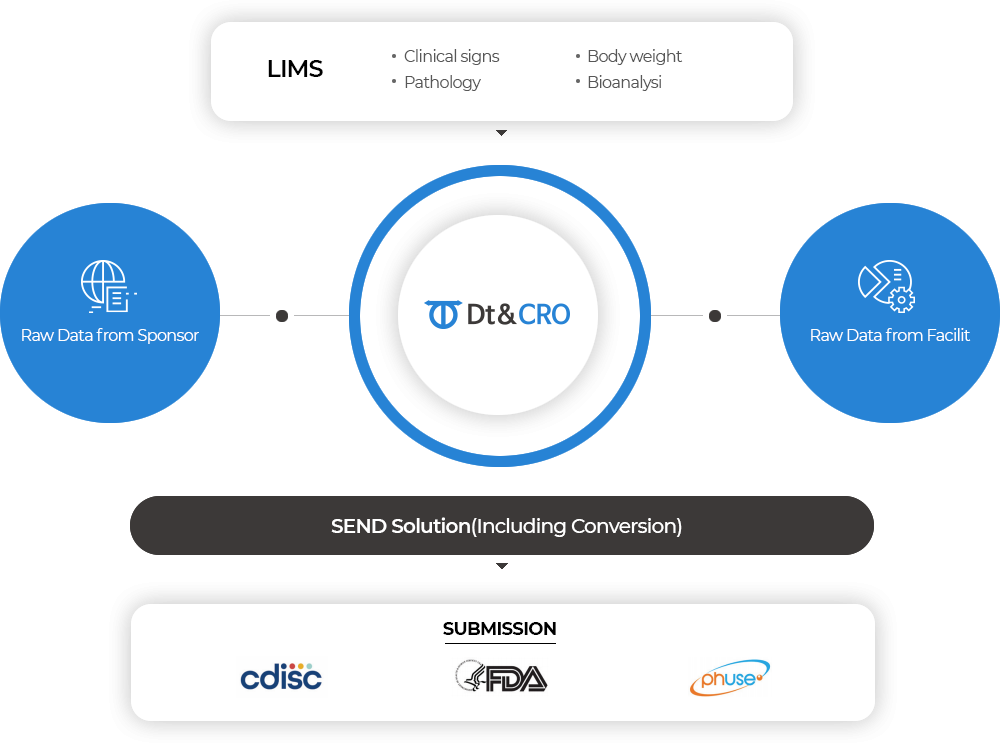
SEND
Standard for Exchange of Nonclinical Data
Meet FDA requirements with SEND, the Standard for Exchange of Nonclinical Data.
We provide not only our own study data but also legacy study data from other test facilities using our own SEND software program.
Submit study data in SEND format, including Final Report, SEND dataset, Define File, and nSDRG, confidently.
Gain regulatory compliance and efficiency for successful drug development.