Dt&CRO
FDA Full Package Service
We dream of future through creative innovation and challenge
SEND
Standard for Exchange of Nonclinical Data
- Big Data Build
- Fast Data Review
- Data Reconfirm
- Efficiency and Convenience
and the FDA’s eCTD requires all nonclinical data to be submitted in SEND format.

-

- SENDIG v.3.0
Single dose, Repeated dose and Carcinogenicity
-

- SENDIG v.3.1
Single dose, Repeated dose and Carcinogenicity
+ Cardiovascular and Respiratory
(Safety pharmacology)
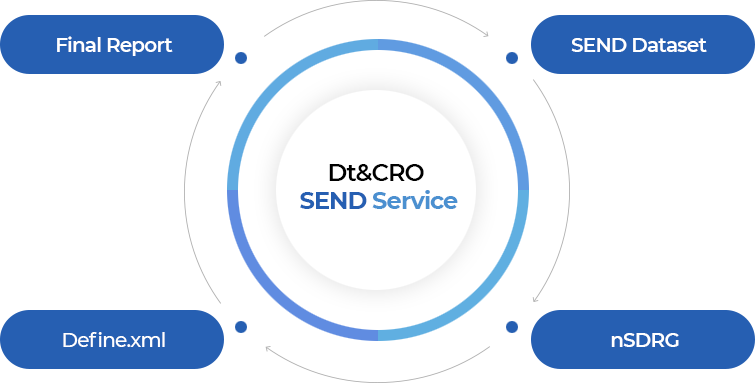
Dt&CRO provides not just dataset conversion but full package of FDA approval with CDISC instructor.
Dt&CRO’s LIMS and SEND Compatibility
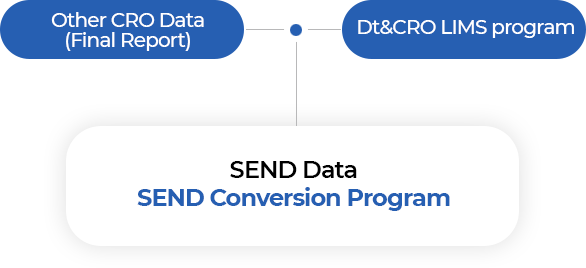
- Dt&CRO SEND conversion
- We provide legacy dataset conversion of data provided by other testing facilities using our in-house SEND conversion program.
- Dt&CRO LIMS synchronization
- Datasets are automatically converted in accordance with existing raw-data, using our own LIMS platform to maintain data consistency.
DT&CRO is the first Korean organization to develop an in-house SEND program,
signing a partnership agreement with the overseas corporation run by a co-author of the CDISC SEND standard.
Our aim is to provide seamless, full-package technology services for domestic
and foreign pharmaceutical and bio ventures for U.S. FDA registration and approval.